What is Optogenetics? - Optogenetics is currently used in neuroscience research as a method that utilizes genetic and optical technologies to activate or deactivate specific cellular behaviors, with high spatial and temporal resolution. Lasers are widely used in Optogenetics both because lasers are coherent and monochromatic sources of light, which means that a very narrow wavelength can be specified and the light output will always be in phase. Also, lasers can be efficiently coupled to optical fibers. This last characteristic is a particularly advantageous in deeper brain structure manipulation with implanted fiber optics.
Diode-pumped solid state (DPSS) lasers with a maximum power of 100mW are an appropriate choice in Optogenetics. The low divergence and high power of the laser beam enables manipulation of activity of single neurons.
The wavelengths used in Optogenetics research span the entire visible spectrum. Depending on the opsins being expressed, an Optogenetics lab may require illumination sources in blue (450-480 nm), green (520-560 nm), yellow (570-600 nm), or red (600-780 nm). Sometimes a combination of wavelengths is used to alternately trigger and silence cellular behaviors. In this case, multi-wavelength lasers are often employed as they enable use of the same beam delivery system while rapidly switching between wavelengths
"Fundamental questions that neuroscientists have previously approached with classical biochemical and electrophysiological techniques can now be addressed using optogenetics. The term optogenetics reflects the key program of this emerging field, namely, combining optical and genetic techniques. With the already impressively successful application of light-driven actuator proteins such as microbial opsins to interact with intact neural circuits, optogenetics rose to a key technology over the past few years."
Dugué, G.P., Akemann, W. & Knöpfel, T. (2012). A comprehensive concept of optogenetics, Progress in Brain Research, 196: 1-28
Generally, optogenetics is a method utilizing both genetic and optical technologies to activate or deactivate specific cellular behaviors, with high spatial and temporal resolution. The fact that optogenetics is primarily used to study the brain is not surprising, as the brain is a highly complex structure exhibiting rapidly-changing behaviours which are often hard to isolate from one another. Optogenetics provides a means of switching neural circuits on and off, at high speed and with little cross-talk. This is a good way to examine what a particular structure does, or how it interacts with other structures. This novel functionality is achieved by binding light-sensitive proteins to the cellular membrane and exciting these molecules with a light source.
The retinal cells in your eyes use proteins called opsins to detect incoming light, which in turn enable you to see. When light of an appropriate wavelength strikes an opsin molecule it changes shape, and through a cascade of reactions this changes the activity of the cell membrane. In the case of retinal cells, the change in opsin configuration leads to the firing of nerve cells in the retina, which send visual information to the brain. This reaction can cycle very quickly, otherwise you would not be able to perceive fast-moving objects. There is a good deal of variation in the cycling rates for various opsins, but the ones used in optogenetics are generally measured in milliseconds. The opsins most commonly used in optogenetics are sourced from microbes, and early work in optogenetics was centered on variants of channelrhodopsin (ChR2), which was originally identified in a green algae called Chlamydomonas.

Optogenetics is unique in that this mechanism can be imparted on cells that normally do not behave in this way. Two common approaches for transferring this behavior are germline transgenesis and somatic gene delivery. Germline transgenesis involves engineering a line of transgenic animals which stably express the opsin proteins in the desired cell types. Somatic gene delivery uses an engineered virus to deliver the genes for opsin expression to an embryonic or mature animal. There are benefits and drawbacks to each technique which are discussed in greater detail on openoptogenetics.org.
Once the opsin is expressed in the desired cells it must be stimulated with light. Each opsin has a slightly different range of wavelengths to which it will respond. In order to isolate the behavior of the target opsin from background effects, a light source with high spectral purity should be used. Ideally, the cell will be illuminated with only the wavelength to which the target opsin will respond, so any change in the cell’s behavior can be attributed to the expressed opsin and not some other photo-activated effect. Lasers are ideal for this reason, as they have an inherently low spectral linewidth (the distribution of wavelengths in the output), high output power, and can be coupled to fiber optics with high efficiency. Research is also being conducted with other light sources, but most suffer from a larger spectral linewidth and greater difficulty in coupling high power levels into optical fiber. (A more detailed discussion of light sources can be found below.)
The wavelengths used in optogenetics research span the entire visible spectrum. Depending on the opsins being expressed, an optogenetics lab may require illumination sources in blue (450-480 nm), green (520-560 nm), yellow (570-600 nm), or red (600-780 nm). Sometimes a combination of wavelengths is used to alternately trigger and silence cellular behaviors. In this case, multi-wavelength lasers are often employed as they enable use of the same beam delivery system while rapidly switching between wavelengths.
Light delivery to the target site can be accomplished in several ways. The simplest approach is to attach the light source directly to the animal. This is straightforward, but the addition of weight to the animal’s head can pose practical issues or raise questions about whether it is influencing their behavior. In restrained animals the light from a laser can be delivered using a scanning mirror which directs the beam to the target site, but this has the disadvantage of requiring the animal to be immobilized and so restricts the types of behaviors that can be investigated. One of the more popular approaches for behavioural research involves using a lightweight optical fiber to deliver the beam to the target.
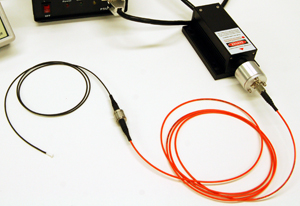
The output end of the fiber optic can be attached to the subject directly, or via a cannula implant which allows for easy attachment and removal of fiber optic cabling. Depending on how much freedom of movement is required, a rotary fiber joint may be installed in-line with the fiber to prevent torquing forces from damaging the fiber or affecting the behavior of the animal. In the case of electrophysiological research where the activity of surrounding cells is of interest (rather than the behavior of the animal) electrode sensors are implanted alongside the fiber optic cable. The choice of light source and beam delivery system is strongly dependent on whether the research is behavioral or electrophysiological in nature.
It has already been shown that this relatively new method enables a better understanding of the structure and function of the brain. The potential implications for psychology, neuroscience, pharmacology, and related fields are significant. At the moment there is so much work being done in optogenetics that it can be challenging to keep up with the current state of knowledge. While the majority of current research is focused on the brain, these techniques can also be used to examine other cellular behaviors for which competing methodologies provide inadequate spatial or temporal specificity. This is a field of study with great opportunity for future advancement, as there is undoubtedly still a lot to learn through the application of light-sensitive switches to cellular behaviors.
Justin Hosaki
Vice President
Arktis Laser
With information from:
1. Dugué, G.P., Akemann, W. & Knöpfel, T. (2012). A comprehensive concept of optogenetics, Progress in Brain Research, 196: 1-28
2. www.openoptogenetics.org
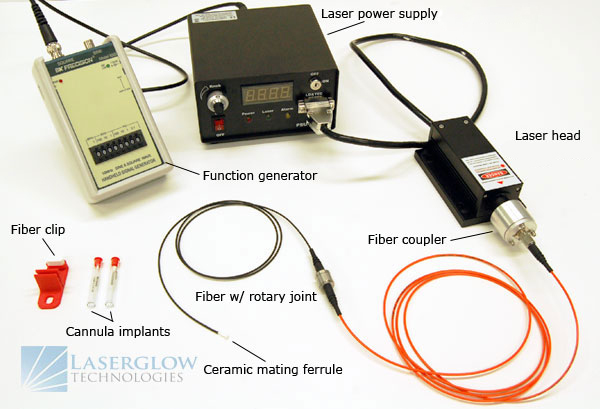
The wavelengths required depend on which actuator opsins are being used. These are usually determined in advance, but in certain cases the choice of opsin may be somewhat flexible and the choice of opsin/wavelength combination becomes a balancing act of cost, convenience, and performance. The wavelengths offered by monochromatic light sources like lasers are limited, and they are not readily available in any arbitrary wavelength. Broad-spectrum tunable versions exist, but these can be very expensive and suffer from limited output power, so it is usually better to choose from discrete wavelengths which are commonly available.
Fortunately, the peak stimulation wavelengths of most frequently-used opsins correlate well to the range offered by solid-state lasers. Channelrhodopsin-2 (ChR2) is stimulated very efficiently by 473 nm, halorhodopsin (NpHR) by 589 nm, and archaerhodopsin (Arch) by 532 nm. Solid-state lasers offering these wavelengths were already well-established in the market before optogenetics became heavily utilized, and increasing demand from the optogenetics research community has motivated the development of lasers which offer compatible wavelengths. While these three wavelengths are applicable to the majority of optogenetics research, Arktis Laser offers laboratory lasers in more than 80 different wavelengths from 266 nm to 2200 nm, so we can support nearly any optogenetics lab regardless of the opsins bring employed.
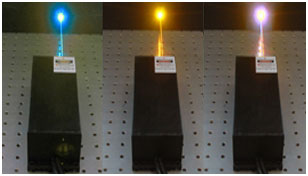
Since an optogenetics experiment will often employ multiple opsins to fulfill various functions, many researchers require a single beam delivery system to carry multiple independently-controlled wavelengths. For this reason, multi-wavelength lasers have become very popular. These are not single laser cavities producing multiple wavelengths, but rather multiple independent laser beams which are combined using dichroic optics. This makes it easy to couple multiple beam lines into a single fiber, which is extremely convenient when activation and silencing must be performed within a short period of time, or when multiple opsins must be stimulated simultaneously.
The power requirement for effective stimulation is usually specified in terms of irradiance, or power per unit area. In optogenetics the most common units for this are mW/mm². Lasers are specified in terms of total output power in Watts, so to figure out how much power you need from the light source it is necessary to work backwards from the required irradiance value. Once the total surface area to be illuminated is known, it is trivial to calculate the total incident power which is required. However, every optical component in the beam delivery system will remove some power from the beam due to absorption, reflection, or scattering.
The transmission ratio of the entire system is the product of the transmission ratios of all components in the beam path, so this can increase quickly as you add components. As an example, if your system consisted of a fiber coupler (coupling efficiency 90%), an optical fiber (attenuation in fiber is negligible), a rotary fiber joint (transmission efficiency 85%), and a mating sleeve (transmission efficiency 80%), the total attenuation coefficient for the system would be 0.90 x 0.85 x 0.80 = 0.61. If you had previously determined that you required 50 mW of total incident power, then the laser source would need to produce at least 50/0.61 = 82 mW.
Because the structure of the beam delivery system will determine this number, it is best to finalize the design of the delivery system (fiber, modulators, cannulae, etc.) before settling on a power level for your light source. Many researchers find that Arktis Laser's Optogenetics Starter Kit is a convenient way to get off the ground quickly when opening a new lab or expanding an existing operation.
It should be noted that more power is not always better. The tissues being stimulated can also be damaged by exposure to high power densities. The damage threshold for these tissues and for the optical components in the beam path should always be considered when determining how much power is appropriate.
When referring to the output power of a laser, the terms “stability” and “noise” have specific meanings which are more-or-less standardized across the industry.
“Stability” refers to long-term output power stability, and is measured as a percent RMS (root mean squared) of the total output power, usually over a time domain of several hours. This indicates the tendency of the output power to drift over time. A laser with a high stability value (e.g. 10%) will fluctuate more than one with the low stability value (e.g. 1%), and this means that your measurements may have greater uncertainty associated with them. Depending on the nature of your experiments your sensitivity to this may vary, and many optogenetics researchers settle on 5% or 3% as an acceptable value. Stability does influence the price of the laser, so once again there is the need to strike a balance between cost and performance.
“Noise” also refers to variation in output power, once again as a percent RMS, but in a much smaller time domain. Noise specifications may be listed in the range of kHz to MHz (i.e. integration times on noise measurements are in the range of ms to ns), and many lasers have different noise characteristics at different frequencies, so noise values may be specified over discrete frequency ranges. Since these fluctuations occur very rapidly, optical noise is usually negligible when conducting behavioral research, but becomes an important consideration for electrophysiological research. Typical noise values for a standard DPSS blue laser may be anywhere from 5-30% RMS at a frequency of 1-20 MHz. For this reason, electrophysiological experiments often employ “low-noise” lasers, which typically guarantee a noise value of <1% or better around a specific frequency range. Low-noise lasers are more expensive than their “standard” counterparts, and some have limitations in their ability to be directly modulated, which are discussed below.
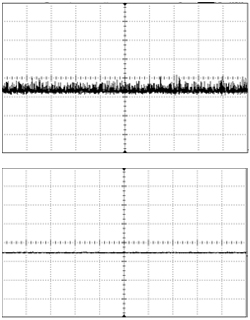
Controlling how light is delivered to the target site is critical for all optogenetics research. For certain types of work this control can be rather coarse, and the light is simply turned on for a defined period and then turned off. More often, the light must be delivered in a train of short pulses, with pulse durations on the order of 5-10 ms and pulse frequencies of 20 Hz or less. (These figures vary widely with the type of experiment being conducted and are by no means comprehensive.) The critical specifications for pulsing behavior are frequency, duration, and temporal pulse shape.
Direct Electronic Modulation
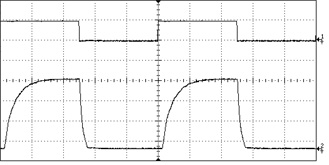
The most basic type of modulation simply turns the laser on and off at the power source. Lasers with embedded electronic modulation have a modulation input (usually a BNC connector), which accepts a signal from a function generator or PLC. This type of modulation can pulse the laser at speeds of up to 20-30 kHz with an arbitrary duty cycle. Diode lasers respond very well to direct modulation, but DPSS lasers tend to exhibit some instability at the moment they are activated, and this can lead to high-frequency artifacts in the pulse shape.
Since this method cycles power to the laser diode, lasers which are sensitive to thermal variation or current level can become unstable when electronically modulated. In particular, low-noise lasers and single-longitudinal-mode (SLM) lasers do not respond well to this kind of modulation and therefore this feature is not offered on these models. In order to modulate a low-noise or SLM laser we recommend the use of an external modulator.
|
Advantages:
|
Disadvantages:
|
External Mechanical Shutters
A variety of mechanical shutters exist which will periodically block or divert the beam. The simplest of these is a beam chopper, which is just a perforated wheel rotating at a fixed speed. Other systems resemble guillotines, irises, rotating mirrors, etc. These systems can be relatively inexpensive, but since they are mechanical in nature there are some limitations on how fast they can operate. Since these devices are external to the laser, you cannot couple the laser directly into fiber, and a free-space fiber coupling system must be installed after the modulator.

|
Advantages:
|
Disadvantages:
|
Acousto-optic Modulators
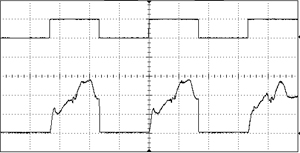
An acousto-optic modulator (AOM, also sometimes called a Bragg cell), is a device which can pulse the laser or alter the transmitted power at a very high frequency. They function by sending the laser beam through a crystal which is subjected to acoustic vibrations. These vibrations alter the refractive index of the material, so the beam can be redirected from the output aperture to a beam dump in an extremely small amount of time. The rise/fall time is limited only by the amount of time it takes for the acoustic wave to traverse the width of the crystal, so this is usually on the scale of nanoseconds. An AOM can modulate a laser with essentially any arbitrary input waveform, with a response frequency in the MHz range. They can be mounted in free-space or installed inline with fiber.

|
Advantages:
|
Disadvantages:
|
The first optogenetics experiments were conducted using lasers. Their high power and monochromaticity make lasers a natural choice for stimulating photoactivated molecules. A laser's highly directional output and low divergence means it is easy to couple into optical fiber with very high efficiency. Lasers still dominate the field based on these technological considerations, and the fact that previous experiments used lasers, so many researchers were hesitant to try different approaches when an effective system had already been established.
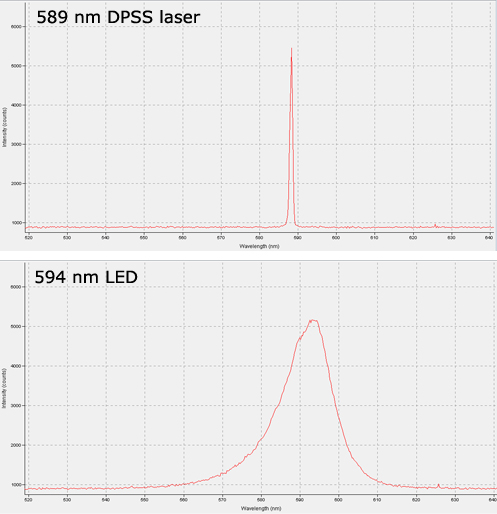
Recent developments in LED technology have made them a viable option for some labs, however. High-powered LEDs are now available in most common optogenetics wavelengths, and for certain types of work they provide some advantages over lasers. The choice of which light source is most suitable will depend on the nature of the research, and as with most choices there are trade-offs on either side. No one light source is superior in all contexts, so it is important to consider the benefits and drawbacks of each before making your decision. Lasers still offer superior output power after fiber coupling, and their low spectral linewidth cannot be matched by LEDs, as illustrated by the spectrogram below.
|
Laser Advantages
|
Laser Disadvantages
|
|
LED Advantages
|
LED Disadvantages
|
| Product | Wavelength | Description | Starting At | In Stock | |
|---|---|---|---|---|---|
 | 445 nm | 445 nm Collimated Diode Laser System | $1,040.00 | Specs |
|
 | 445 nm | 445 nm Collimated Diode Laser System with Near-TEM00 Beam | $2,583.00 | Specs |
|
 | 447 nm Best Seller! | 447 nm Collimated Diode Laser System 200 - 1000 mW Output Power | $1,417.00 | Specs |
|
 | 447 nm Best Seller! | 447 nm Collimated Diode Laser System 4000 - 20000 mW Output Power | $9,998.00 | Specs |
|
 | 450 nm | 450 nm Collimated Diode Laser System with Near-TEM00 Beam 5 - 50 mW Output Power | $2,583.00 | Specs |
|
 | 462 nm | 462 nm Collimated Diode Laser System 5 - 800 mW Output Power | $1,990.00 | Specs |
|
 | 470 nm Best Seller! | 470 nm Collimated Diode Laser System 100 - 1500 mWOutput Power | $3,616.00 | Specs |
|
 | 473 nm Best Seller! | 473 nm DPSS Laser System 5 - 100 mW Output Power | $1,664.00 | Specs |
|
 | 473 nm Best Seller! | 473 nm DPSS Laser System 200 - 500 mW Output Power | $2,482.00 | Specs |
|
 | 473 nm Best Seller! | Environmentally Sealed 473 nm DPSS Laser System 10 - 100 mW Output Power | $4,225.00 | Specs |
|
 | 473 nm Best Seller! | Fanless 473 nm DPSS Laser System 5 - 500 mW Output Power | $4,805.00 | Specs |
|
 | 473 nm Best Seller! | 473 nm DPSS Laser System 800 - 1500 mW Output Power | $8,673.00 | Specs |
|
 | 473 nm Best Seller! | 473 nm DPSS Laser System | $13,975.00 | Specs |
|
 | 473 nm Best Seller! | 473 nm DPSS Laser System - mW Output Power | $20,150.00 | Specs |
|
 | 532 nm Best Seller! | 532 nm DPSS Laser System 5 - 300 mW Output Power | $1,437.00 | Specs |
|
 | 532 nm Best Seller! | Environmentally Sealed 532 nm DPSS Laser System 10 - 300 mW Output Power | $4,668.00 | Specs |
|
 | 532 nm Best Seller! | 532 nm DPSS Laser System 3000 - 5000 mW Output Power | $6,958.00 | Specs |
|
 | 532 nm Best Seller! | 532 nm DPSS Laser System 8000 - 20000 mW Output Power | $11,375.00 | Specs |
|
 | 561 nm | Fanless 561 nm DPSS Laser System 5 - 200 mW Output Power | $3,600.00 | Specs |
|
 | 561 nm | 561 nm DPSS Laser System 1500 - 2000 mW Output Power | $19,105.00 | Specs |
|
 | 589 nm Best Seller! | 589 nm DPSS Laser System 10 - 50 mW Output Power | $5,913.00 | Specs |
|
 | 589 nm Best Seller! | 589 nm DPSS Laser System 100 - 300 mW Output Power | $7,954.00 | Specs |
|
 | 589 nm Best Seller! | Environmentally Sealed 589 nm DPSS Laser System - mW Output Power | $11,375.00 | Specs |
|
 | 589 nm Best Seller! | 589 nm DPSS Laser System 1000 - 3000 mW Output Power | $17,982.00 | Specs |
|
 | 640 nm Best Seller! | 640 nm Collimated Diode Laser System 5 - 200 mW Output Power | $2,583.00 | Specs |
Here are a list of research papers that reference Arktis Laser products in their optogenetics.